A pioneering initiative by Controllab increases safety in the use of these devices and enhances the reliability of the reports.
POCTs are part of the in vitro diagnostic (IVD) product category and include reagents, substrates, buffer solutions, and other consumables necessary for analysis.
Although they are designed to be accurate, it is essential to validate them and use quality control to monitor the analytical performance of these devices, ensuring the reliability and effectiveness of the tests.
Brazil’s regulatory framework, RDC 786/2023, expanded the use of these POCTs, which were already used in laboratories, collection centers, and hospitals, to also be used in clinics, medical offices, and pharmacies.
With the expansion of the Brazilian market, new POCTs have been introduced, broadening the range of tests available in this modality.
Consistency in POCT batches
Controllab, the largest provider of quality control solutions in Latin America, with its unique expertise in data analysis, has highlighted significant variations in the results of POCTs from different batches of the same manufacturer in its Proficiency Testing, also known as External Quality Assessment (EQA).
In light of this situation, the company undertook several initiatives to improve the quality of these results. However, with the growing use of POCTs, Controllab identified the need for a more robust approach to ensure greater reliability and safety in the batch-to-batch variation of these devices.
This new initiative aims to identify which POCTs maintain consistency across their batches, ensuring higher quality in the analytical process for laboratories and other clinical analysis establishments.
Committed to its mission of helping clients provide accurate and indisputable services, Controllab has launched a program that certifies the quality of performance across POCT batches distributed in Brazil.
Quality certification for POCT
Quality control allows for monitoring that the POCT, along with its reagents and consumables, maintains its properties and effectiveness. This involves performance evaluation testing.
Among quality control practices, it is essential to ensure that the replacement of reagent batches does not affect the analytical routine. To enhance reliability in this assessment, Controllab offers the Batch-by-Batch Certification Program.
In this program, the batches of these products are evaluated according to relevant guidelines and literature, using representative materials from routine use. The goal is to verify if the results between batches are consistent and maintain analytical performance.
Advantages of using batches certified by the Program
By using batches certified by Controllab’s Batch-by-Batch Certification Program, your establishment benefits from a prior evaluation of the batch integrated into the routine, comparing its performance to the previous batch.
The certificate ensures that the batch in use is consistent with previous batches from the same manufacturer, increasing the reliability of the analyses. This certification introduces efficiency and cost savings for your establishment, promoting:
- Quality and reliability: Prevents potential issues or deviations that could affect the performance of the devices.
- Routine simplification: With certification, the integration of a new batch into analytical processes becomes simpler and more efficient.
- Cost and resource reduction: Saves on reagents, time, and effort required for performance testing and analysis between batches, optimizing the frequency of Internal Quality Control.

How to identify batches validated by the Program?
The verification is evidenced by the certificate issued by Controllab. The manufacturer or the holder of the POCT registration provides the certificate to the establishments. Additionally, the certificate can be checked directly on Controllab’s website, on the program page, or through a QR code present on the certificate itself, ensuring the authenticity of the information.
Manufacturers that have already certified their quality
At the forefront of demonstrating the quality of their POCTs, manufacturers Abbott, Biosys, and MedLevensohn have already secured certification for a selection of their products.
These manufacturers meet the needs of their audience by helping to reduce costs and efforts in monitoring the replacement of new reagent batches in the analytical routine. This ensures confidence in their products. Check out the batches that have already received program certification:
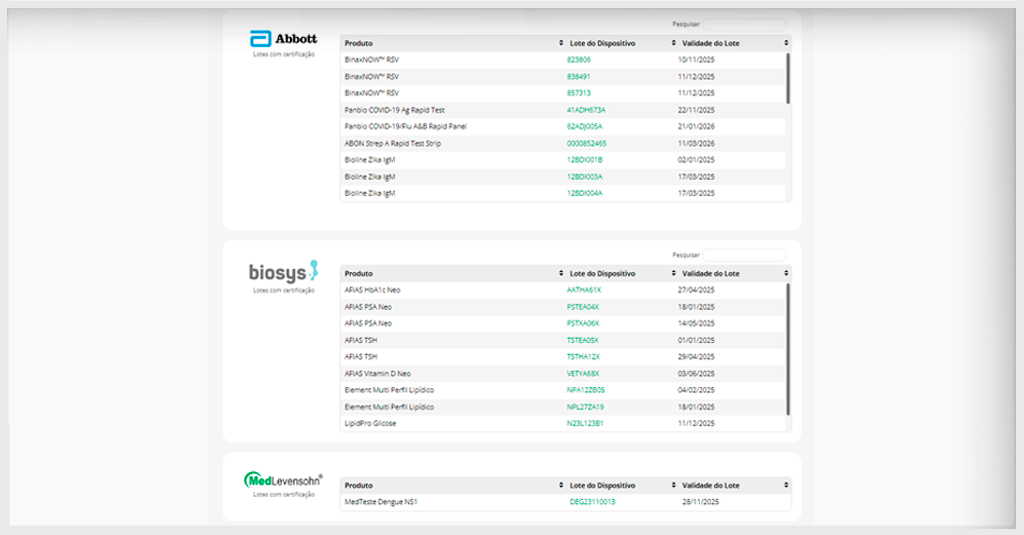
To view the updated list of certified batches, please visit this link.
The Batch-by-Batch Certification Program is an innovative initiative for the segment in Brazil. This pioneering effort is a hallmark of Controllab, known for its competence, experience, trust, and innovation. The company is recognized for adhering to major standards related to its operations: ISO 9001, 17025, 17034, and 17043.
For more information about the Batch-by-Batch Certification Program, manufacturers and clinical analysis establishments can access this exclusive channel and speak directly with the specialist for this solution.
